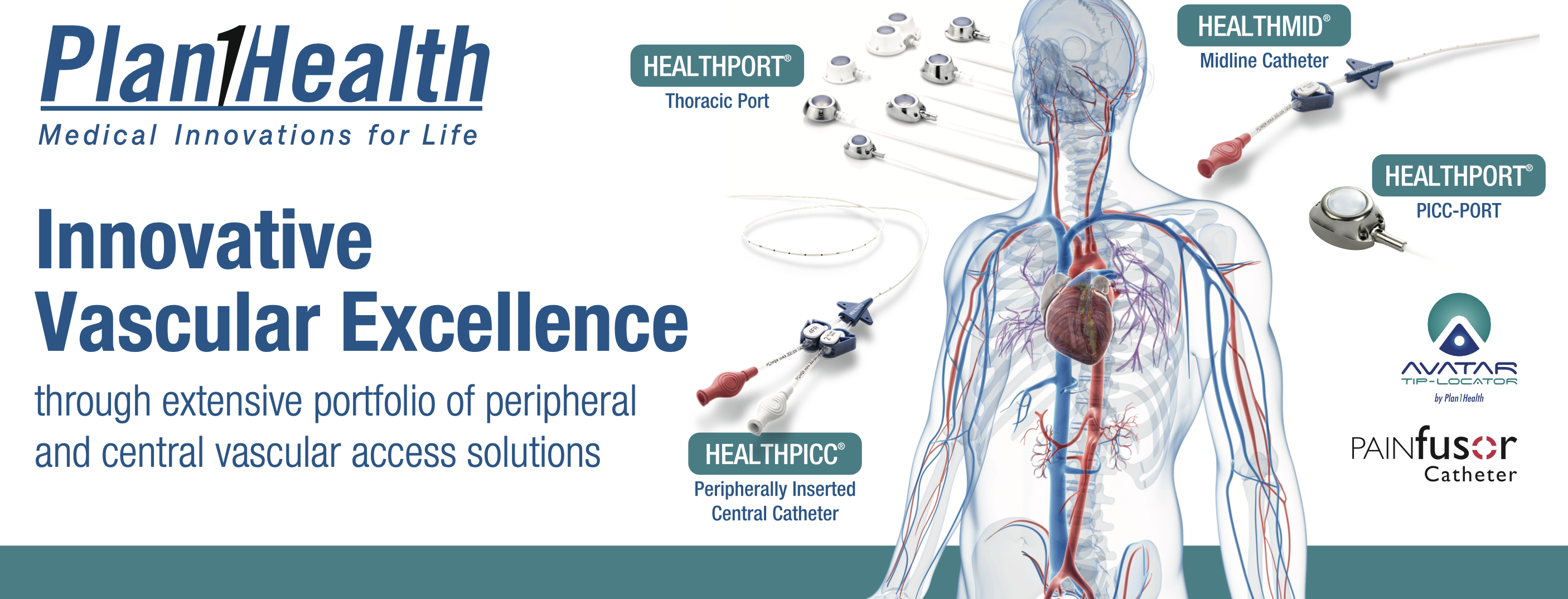
Our Partner's History
Founded in 1990, Plan 1 Health develops, manufactures and markets long-term implantable medical devices in the human body, including systems for vascular access and for the infusion of drugs, sold in Italy and abroad. The commercial and administrative office includes the warehouses of finished products and semi-finished products, an ISO 7 class Clean Room, a laboratory for washing and surface treatment, a production area dedicated to quality control, assembly, packaging and to labelling. Plan 1 Health is able to fully follow the development of a medical product, from idea and design development to engineering, from certification to mass production. The entire development of the project is conducted according to international standards.